WVU Heart and Vascular Institute is now offering protected TAVR
MORGANTOWN, W.VA. -- The WVU Heart and Vascular Institute is one of the first in the country to introduce new technology shown to protect patients from the risk of stroke during minimally invasive heart valve surgery, known as transcatheter aortic valve replacement (TAVR).
TAVR is a relatively new approach to treating aortic stenosis. During the procedure, a team consisting of interventional cardiologists and a cardiac surgeon makes a small incision in the groin area and replaces the valve via a catheter. This approach is especially beneficial to patients who are not deemed to be ideal candidates for open heart surgery.
However, the majority of patients undergoing TAVR have severe valvular calcifications and frail plaque materials on the aorta. These materials can be dislodged during the procedure and may then travel to the brain and cause a stroke. Because of this, the WVU Heart and Vascular Institute is implementing protected TAVR using the Sentinel Cerebral Protection System, the first FDA-cleared device available in the U.S. to capture and remove this debris before it reaches the brain. The device has been shown to reduce strokes by 63 percent during the procedure and in the first 72 hours after it, when most strokes occur.
In a protected TAVR procedure, the Sentinel system is delivered first via a small tube inserted through a small puncture in the right wrist. Using a catheter, two filters are placed in the two main arteries between the heart and brain. Those filters collect debris throughout the procedure, preventing it from traveling to the brain. When the procedure is complete, the filters and collected debris are removed from the patient.
“TAVR technology has revolutionized the treatment of severe aortic stenosis. However, the risk of procedural stroke has been a major pitfall of the procedure. The Sentinel device is the first approved device that has been shown to significantly reduce the risk of stroke during TAVR,” Mohamad Alkhouli, M.D., director of structural heart interventions at the WVU Heart and Vascular Institute, said. “We are proud to be among the first centers to offer this novel device to our TAVR patients”.
The initial clinical trial of the device included 19 centers across the U.S. and Germany and showed that it captured debris in 99 percent of TAVR cases. The device was then made available to 10 additional sites, of which the WVU Heart and Vascular Institute is one.
“The introduction of embolic protection devices during TAVR is symbolic of our commitment toward combining cutting-edge health innovation with highest safety standards for modern cardiac procedures In West Virginia,” Partho Sengupta, M.D., chief of cardiology at the WVU Heart and Vascular Institute, said.
To date, more than 3,500 patients worldwide have undergone the protected TAVR procedure.
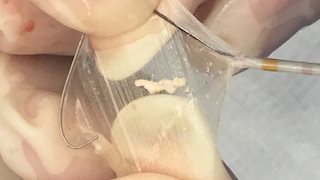
“Since the Sentinel Cerebral Protection System was recently made available to the WVU Heart and Vascular Institute, we have employed it routinely in every patient undergoing TAVR. We successfully deployed the device in 23 of 24 procedures. Excessive tortuosity in the neck arteries prevented the one device from being deployed. In most cases, we can predict the ability to position the Sentinel system from a CT scan obtained routinely before the TAVR procedure. We observed debris in the filters in more than 50 percent of the cases, including the large piece of plaque pictured, which would most certainly have caused a significant stroke,” Bryan Raybuck, M.D., director of the cardiac catheterization lab at the WVU Heart and Vascular Institute, said.
“The entire heart team at WVU is honored to be able to offer this groundbreaking technology to our patients for the reduction in stroke risk associated with TAVR.”